
While working on medication management for the elderly, we stumbled upon an equally urgent concern, this time at the other end of the age spectrum. We found ourselves asking: Are our medicines packaged safely enough for children?
The answer was sobering. In most Indian households, they’re not.
Despite being life-saving for adults, many commonly used medications can cause serious harm , even death, if accidentally ingested by a child. Yet, nearly all medicines in India are packaged in formats that are easy to open, not just for adults but for children too.
This is more than a packaging oversight. It’s a widespread yet preventable risk and one that’s barely being talked about.
The Reality: Accidental Poisoning Is Far More Common Than We Think
The data paints a clear and deeply concerning picture. In a hospital-based study from Delhi, 75% of poisoning cases involved children under five, and 17% of these were caused by accidental ingestion of medicines, making it the single most common cause of childhood poisoning in that setting.
Zooming out to a national level, research published in the Indian Journal of Pediatrics estimates that over 90% of childhood poisoning cases in India result from accidental ingestion of medicines. These numbers are not isolated. They're echoed by doctors in emergency departments who regularly see children brought in after consuming adult medications, painkillers, iron tablets, antihistamines, even heart medications left within reach at home.
These incidents cut across socio-economic backgrounds, urban and rural alike.
Why Does Unsafe Packaging Remain the Norm?
For something so high-risk and avoidable, it’s natural to ask, Why hasn’t this changed?
There are a few overlapping reasons:
- No regulatory mandate: India’s Drugs & Cosmetics Act does not require medications to be sold in child-resistant packaging. As a result, manufacturers are not obligated to change their practices.
- Low public awareness: Most caregivers don’t know what child-resistant packaging (CRP) is or that it even exists and therefore can’t demand it.
<aside>Child-resistant packaging (CRP), also known as special packaging, is designed to be difficult for young children to open, while still being relatively easy for adults to use. This type of packaging is crucial for products that could be harmful if ingested, such as medications, household chemicals, and certain pesticides. The goal is to reduce the risk of accidental poisoning in children under five years of age.
</aside>
- Export vs. domestic standards: Ironically, Indian pharmaceutical companies do manufacture CRP-compliant packaging but mostly for export markets. For medicines sold in India, such safety features are largely absent.
- Lack of perceived urgency: In the absence of industry pressure or consumer demand, there’s limited momentum to act.
In short, it’s a mix of regulatory gaps, market inertia, and public unawareness; a dangerous combination when it comes to child safety.
What Happens When Child Resistant Packaging (CRP) Is Taken Seriously?
Fortunately, there are global examples that show what’s possible when child safety becomes a priority.

When the United States passed the Poison Prevention Packaging Act in 1970, which made child-resistant packaging mandatory for household drugs, poisoning-related deaths among children dropped by nearly 45% over the next decade.
Similar mandates now exist in the UK, Australia, the EU, and several other countries. These policies are backed by strict enforcement and public awareness campaigns, making CRP the default, not the exception.
The impact isn’t just limited to fewer hospital visits. CRP also builds long-term public trust in medication safety, reduces caregiver anxiety, and strengthens pharmaceutical reputation.
India isn’t behind on technology or materials, just policy.
What’s Already Possible in India
The good news is: India already has the capabilities to roll out CRP solutions at scale.
Companies like Bilcare Research are already manufacturing child-resistant blister foils and cap systems for clients overseas. Products like Crispak PP meet international CRP standards and are being used for export to highly regulated markets.
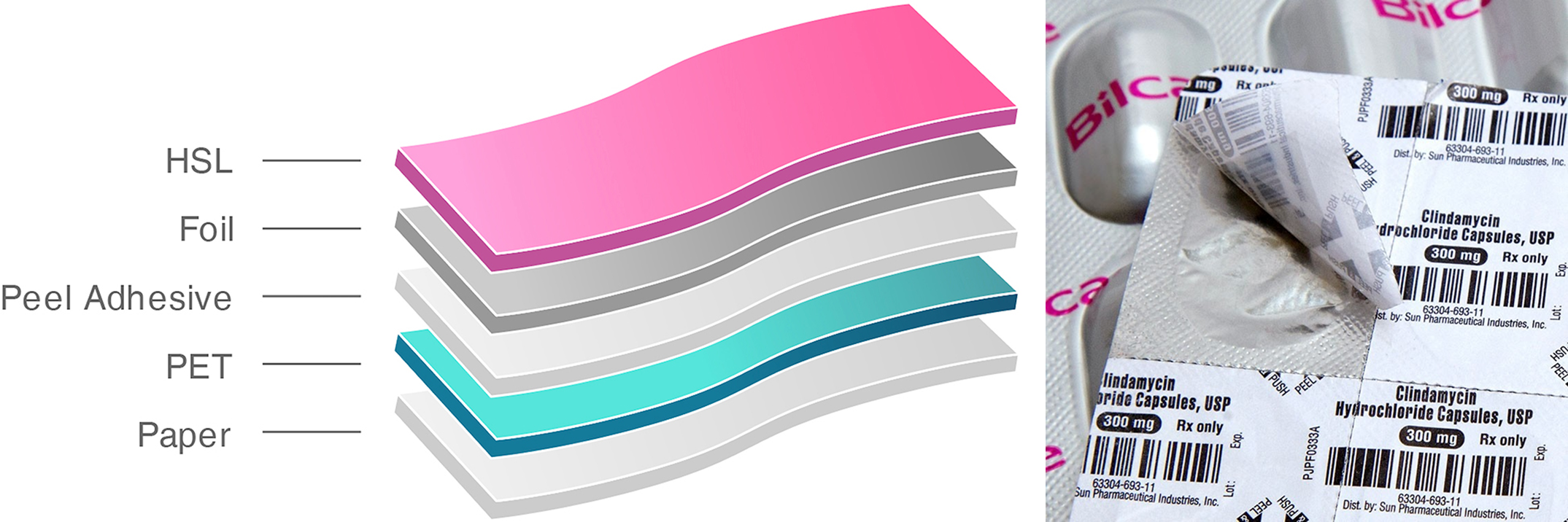
Indian packaging innovators are not lacking in technology or design, they’re already part of the global supply chain for safety-first solutions.
But within India, these same innovations are barely being used.
What’s missing is a clear policy direction and incentive structures that reward early adopters. In parallel, public health campaigns can help shift consumer awareness, making caregivers more likely to ask: Is this safe for my child?
So, What Needs to Change?
To make CRP mainstream in India, we don’t need to start from scratch just shift priorities:
- Introduce regulatory mandates: Begin with high-risk medications those commonly used in households and particularly harmful in small doses (like iron, cardiac, or antidepressant drugs).
- Encourage voluntary adoption: Until policy catches up, Pharma companies can lead by example, showing that safety-first packaging can coexist with profitability.
- Support local manufacturers: Recognise and promote Indian packaging firms already producing CRP for exports.
- Drive public awareness: Caregivers, paediatricians, and pharmacists all need to be part of the conversation. If people don’t know this is a risk or that a safer alternative exists, they won’t demand better.
Change doesn’t always come from regulation alone. It can start from small shifts, a Pharma company launching a pilot CRP line, a design firm experimenting with safer formats, or a public health nonprofit raising awareness in schools and clinics.
Have you come across efforts in India, small or large, that are already pushing the needle on child-safe packaging?





-min.png)


.avif)









